In the fast-paced world of medical innovation, the demand for quicker access to safe and effective treatments continues to grow. Traditional clinical trials, though essential for ensuring safety and efficacy, often follow a rigid, stepwise process that can take years to complete. As the healthcare community seeks ways to accelerate drug development without compromising quality, one approach has garnered considerable attention: combined-phase clinical trials.
What Are Combine Phase Clinical Trials?
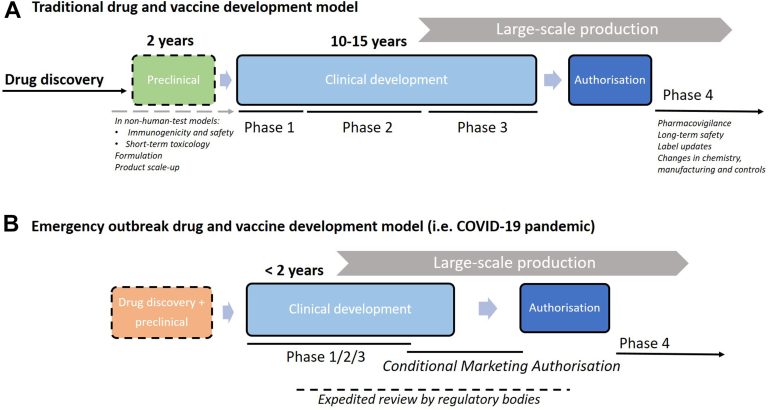
Clinical trials have long been the cornerstone of medical advancements, providing a meticulous and systematic pathway to evaluate new drugs and interventions. Traditionally, clinical research is organized into separate phases: Phase I tests basic safety and dosing in a small group of healthy volunteers; Phase II expands the testing to more individuals to determine efficacy and further assess safety; and Phase III, often the largest, compares the new intervention to current standards in diverse populations. However, the traditional step-by-step progression of these phases can be slow, sometimes becoming a barrier for patients who urgently need new treatments.
Enter combine phase clinical trials, which are rapidly gaining favor among scientists and pharmaceutical sponsors. These studies merge two phases—such as Phase I/II or Phase II/III—into a single, continuous protocol. This innovative approach enables researchers to collect more comprehensive data by aligning the objectives of each phase, thereby improving efficiency from the earliest safety assessments to the initial signals of efficacy. By bridging the gap between phases without unnecessary interruption, combined phase trials support faster decision-making about whether a drug is likely to benefit patients, often shaving months or even years off the development process.
Key Benefits of Combine Phase Trials
- Streamlined Development Timelines:By combining phases, researchers can avoid the administrative delays that often occur between traditional trial steps. This streamlined progression is particularly beneficial when early results indicate a strong potential for a new therapy, allowing teams to move forward without waiting for separate approval to initiate the next phase. According to global studies, such designs can reduce development time by up to 30% compared to sequential phases.
- Consistent and Comprehensive Data:Combining phase trials keeps participants under observation for a more extended, uninterrupted period, leading to higher-quality data and fewer gaps. This consistency enables scientists to understand better how patients respond to various doses over time, providing valuable insights for adjusting ongoing treatments and optimizing outcomes.
- Increased Patient Access and Engagement: For patients with limited options, merged-phase studies can offer earlier access to promising experimental medicines. This approach has proved especially crucial in fields with high unmet needs—such as oncology or rare diseases—where every day counts.
Recent responses to emerging public health crises have showcased the value of this model. For example, vaccines and treatments developed during pandemics have leveraged combined-phase clinical trials, enabling global regulators to review and authorize therapies in record time without compromising essential safeguards.
How Combine Phase Trials Shape Future Medicines
The impact of combined-phase clinical trials is already visible in how they accelerate breakthroughs for patients facing urgent medical needs. According to the National Library of Medicine, these trials enable researchers and sponsors to assess preliminary results and adapt study protocols quickly, facilitating agile development and more personalized approaches to medicine. Early “go/no-go” decision points enable the discontinuation of unsuccessful treatments without unnecessary delay, thereby saving considerable time and resources for all stakeholders involved.
According to a recent analysis of combination clinical trials, this flexibility has led to more targeted drug development efforts, particularly for rare diseases that might otherwise be overlooked by cumbersome traditional research cycles. In oncology, for example, combined phase trials help scientists swiftly move promising therapies to larger, more definitive studies, potentially changing the standard of care for life-threatening conditions.
The Regulatory Perspective
The regulatory environment is evolving to keep pace with innovation in trial design. Agencies like the FDA and EMA are increasingly open to creative approaches that uphold rigorous standards for patient safety and evidence quality. For sponsors, the key is to approach regulators early—ideally during the study design stage—to align expectations and ensure that data from combined-phase clinical trials are presented, interpretable, and actionable.
Regulatory flexibility does not mean a relaxation of the high bar for efficacy and safety. Instead, it opens doors for novel trial structures that can bring effective treatments to the public more swiftly, provided that clear endpoints, risk mitigation plans, and robust monitoring are built into the combined study protocols.
Patient Involvement in Combine Phase Trials
Patients are increasingly recognized as central partners in clinical research, especially as trial designs become more innovative. According to BMC, engaging advocacy groups early in the process helps ensure protocols are patient-centered, accessible, and transparent. Their input can refine study objectives, reduce burdens, and address genuine concerns about risks and benefits, emphasizing patient involvement as a key factor in improving trial relevance and participant experience.
For individuals considering enrollment in a combined phase trial, open communication and education are essential. Participants gain the unique opportunity to access treatments sooner and contribute to important scientific discoveries. At the same time, clear patient information highlights safety measures, realistic outcomes, and avenues for ongoing dialogue with research teams, critical elements that strengthen trust and motivation.
Recent Success Stories in Combine Phase Trials
There are now several examples where combined-phase clinical trials have contributed to crucial advancements in healthcare. Over the last decade, specific groundbreaking cancer therapies, novel antivirals, and treatments for rare diseases have reached patients more quickly by leveraging this approach. Real-world evidence continues to show that combining phases with careful planning and adaptive study design can cut down both time and costs.
Insights from leading researchers emphasize that, according to industry perspectives on efficiency, merged trials help sponsors gather rich data and speed up vital decisions. As a result, patients benefit from accelerated innovation without compromising scientific rigor or safety standards.
Steps for Sponsors Considering Combine Phase Trials
- Early Regulatory Engagement: Initiate discussions with relevant agencies at the earliest stage of planning to clarify trial objectives, data requirements, and safety expectations.
- Strategic and Multidisciplinary Design: Assemble a team of clinical researchers, data scientists, and patient advocates to craft robust and flexible protocols that anticipate challenges unique to combine phase trials.
- Transparent Patient Communication:Integrate patient and advocacy group feedback into every stage and maintain open lines of communication about the distinctiveness, benefits, and risks involved in merged-phase participation.
Combining phase clinical trials represents a forward-thinking model for delivering the right medicines to those who need them in a shorter timeframe, with the same high standards the field demands. Their rising prominence signals a new era in drug development, one where collaboration, efficiency, and patient-centricity underpin every breakthrough.